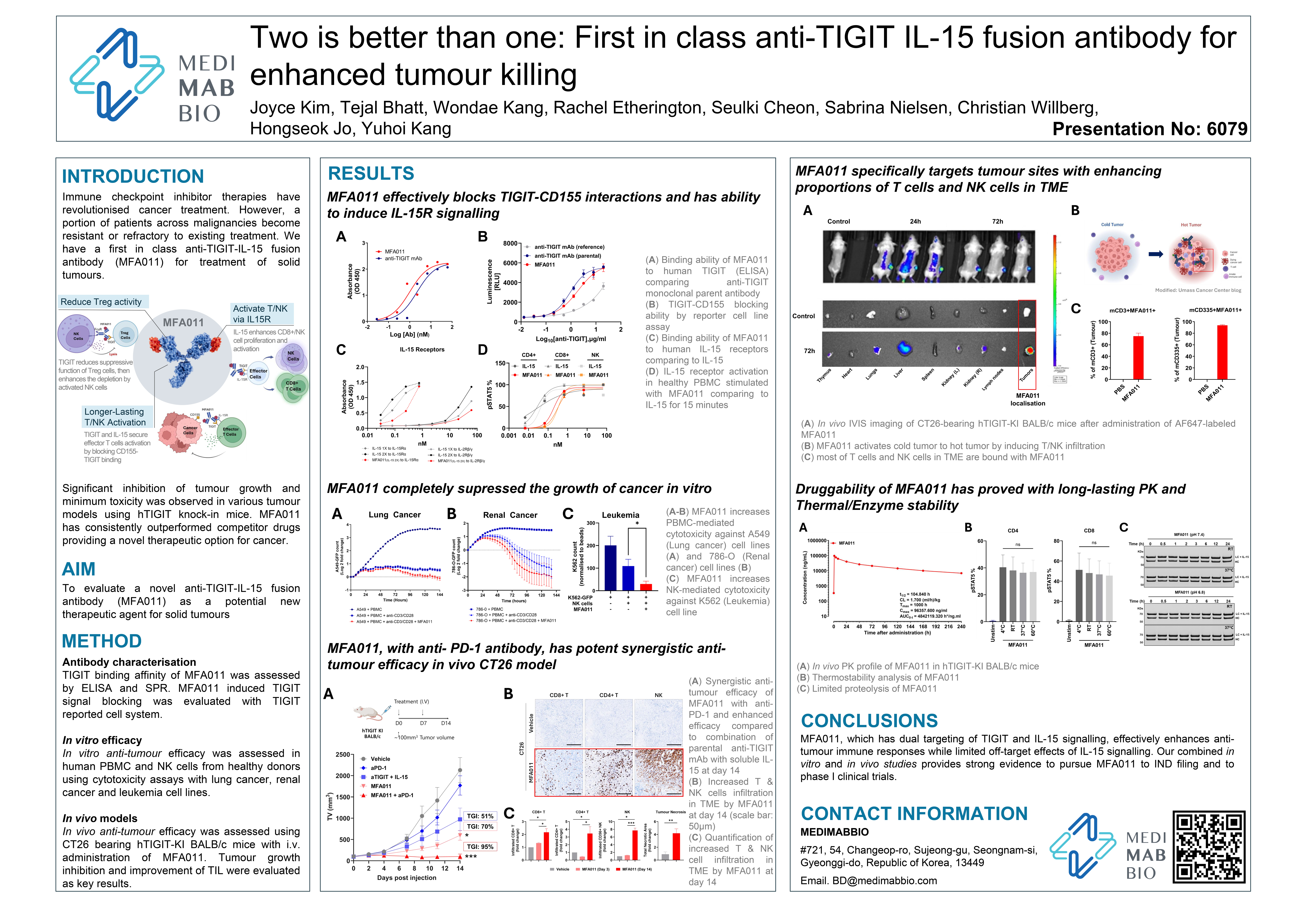
MediMabBio successfully presented promising preclinical results for its aTIGIT-IL15 fusion antibody, MFA011, at the AACR 2025 Annual Meeting held in Chicago In preclinical models, MFA011 demonstrated enhanced anti-tumor efficacy and reduced immunotoxicity, highlighting its potential as a novel immuno-oncology therapeutic.
MFA011 uniquely integrates TIGIT immune checkpoint blockade with IL-15-mediated immune activation, resulting in prolonged signaling, increased activity of NK and CD8+ T cells, and selective depletion of regulatory T cells (Tregs). This mechanism offers a distinct therapeutic profile compared to conventional TIGIT-targeting agents.
At the conference, MediMabBio also engaged with leading global biopharma companies and key opinion leaders (KOLs) to share its research findings and explore strategic collaboration opportunities.
These results mark a key milestone for MediMabBio’s proprietary fusion antibody platform, providing strong momentum for the clinical development of MFA011 and reinforcing the company’s growing presence in the immuno-oncology field.